Table of Contents
Cocaine is a well-known stimulant drug that comes from the leaves of the coca plant. While it’s often talked about because of its use and abuse, it also has an interesting chemical makeup. In chemistry terms, the cocaine chemical formula is C₁₇H₂₁NO₄. This formula stands for a compound of 17 carbon atoms, 21 hydrogen atoms, one nitrogen atom, and four oxygen atoms. Even though it’s a natural substance, it has a complex chemical structure that gives it powerful effects on the human body.
What Does the Cocaine Formula Mean?
When you see C17H21NO4, it might look like a random mix of letters and numbers. However, each part of this formula tells us something about how the molecule is built.
- C17 means the molecule has 17 carbon atoms.
- H21 shows it has 21 hydrogen atoms.
- N stands for one nitrogen atom.
- O4 indicates there are four oxygen atoms.
Together, these elements form a special kind of organic compound known as an alkaloid. Cocaine belongs to a group of alkaloids called tropane alkaloids, which have effects on the nervous system.
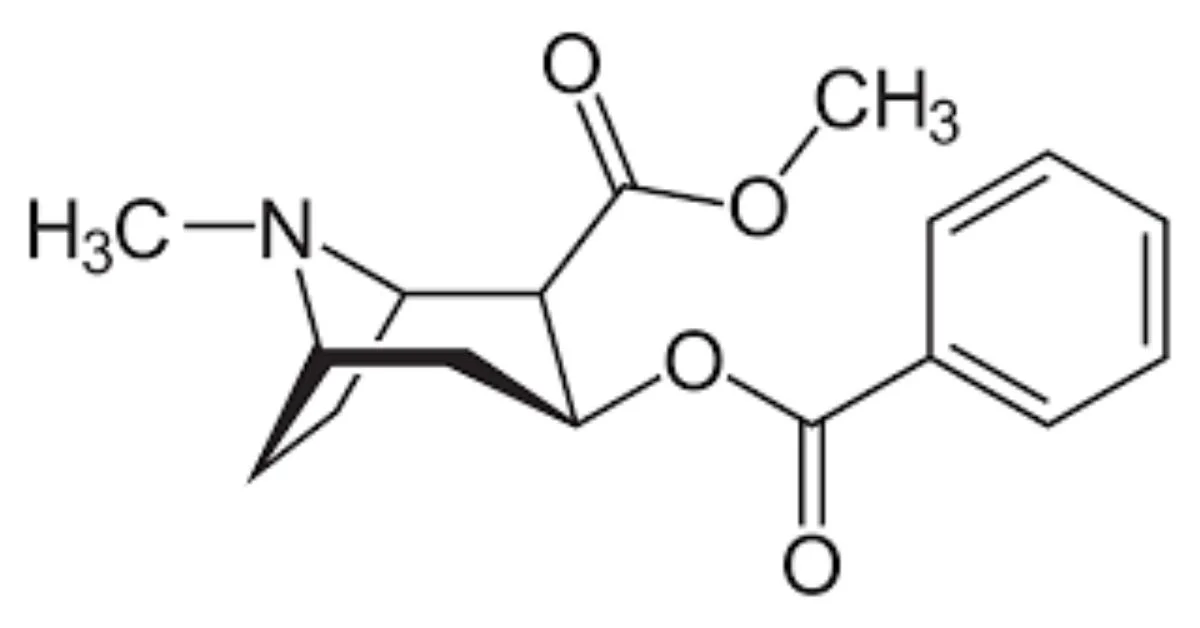
Cocaine’s Structure Explained Simply
Cocaine’s structure is a bit like a 3D puzzle. At the center of the molecule is a tropane ring, which is a circular shape made of carbon and nitrogen atoms. Attached to this ring are different chemical groups that give cocaine its specific effects:
- A benzoyl group, which contains a benzene ring and makes the molecule more fat-soluble, helping it enter the brain quickly.
- A methyl ester group, which plays a role in how the molecule behaves in the body.
- A hydroxyl group (–OH), which helps determine how the molecule interacts with water and other substances.
This unique structure allows cocaine to block certain proteins in the brain that reabsorb neurotransmitters like dopamine, leading to the intense effects users feel.
Physical and Chemical Properties of Cocaine
Here are some important properties of cocaine that scientists and medical professionals look at:
- Molecular Weight: About 303.35 grams per mole.
- Appearance: In its pure form, cocaine is a white crystalline powder. When processed into “crack cocaine,” it takes on a more rock-like shape.
- Solubility: Cocaine hydrochloride dissolves well in water. This is the form often used medically. Freebase cocaine doesn’t dissolve in water but can be smoked.
- Melting Point: Pure cocaine melts at around 98°C (208°F).
- Boiling Point: Because it breaks down before boiling, cocaine doesn’t have a true boiling point under normal pressure.
These properties help forensic scientists identify the drug and also determine how it behaves in the body or when used in different ways.
Natural Source of Cocaine
Cocaine is not made in a lab by default. It’s found naturally in the leaves of the Erythroxylum coca plant, which grows in South America. Indigenous people have used coca leaves for centuries, chewing them to reduce hunger and fight fatigue. The chemical we know as cocaine is isolated and refined from these leaves in a multi-step process involving acids and solvents. The final result is a much more concentrated and potent substance than the natural form.
Medical and Scientific Uses of Cocaine
Believe it or not, cocaine does have some medical uses. In small doses and under strict medical supervision, it’s used as a local anesthetic, especially during surgeries involving the nose and throat. It works well in these cases because it both numbs the area and constricts blood vessels, reducing bleeding.
However, because of its addictive nature and potential for misuse, it is rarely used today. Safer and more effective drugs like lidocaine and benzocaine are usually preferred in modern medicine.
How Cocaine Affects the Body?
When someone uses cocaine, the drug quickly reaches the brain and changes how nerve signals are sent. It blocks the reabsorption of dopamine, a chemical that helps regulate pleasure and movement. This causes dopamine to build up in the brain, creating feelings of euphoria, increased energy, and confidence.
But these effects come with serious risks. Over time, the brain gets used to the drug, leading to tolerance, dependence, and addiction. Long-term use can damage the heart, brain, and other organs.
Freebase vs Cocaine Hydrochloride
Cocaine comes in two main forms:
- Cocaine hydrochloride: This is the powdered form, often snorted or injected. It’s water-soluble and used occasionally in medicine.
- Freebase cocaine (often called “crack”): This form is smokable. It doesn’t dissolve in water and produces more immediate but short-lived effects.
The chemical formula (C17H21NO4) remains the same in both forms, but their physical and chemical behaviors differ depending on how they’re prepared.
How is Cocaine Detected?
Cocaine and its byproducts, especially benzoylecgonine, can be detected in the body using urine, blood, saliva, or hair samples. Drug tests usually focus on these breakdown products, which can stay in the body for days or even weeks, depending on the amount used and the person’s metabolism.
In forensic labs, scientists use tools like gas chromatography-mass spectrometry (GC-MS) to confirm the presence and identity of cocaine. These techniques are very accurate and are often used in legal or criminal cases.
Summary Table
| Property | Description |
|---|---|
| Chemical Formula | C17H21NO4 |
| Molecular Weight | ~303.35 g/mol |
| Source | Coca plant (Erythroxylum coca) |
| Form | Freebase or Hydrochloride salt |
| Appearance | White powder or crystalline rocks |
| Medical Use | Local anesthetic (rare today) |
| Main Effects | Euphoria, alertness, increased heart rate |
| Detection | Urine/blood/hair drug tests |
Conclusion
The cocaine chemical formula, C17H21NO4, represents much more than just a series of atoms. It reflects a complex molecule that has powerful effects on the human body, both good and bad. While it has limited medical use, it’s mostly known for its role as an illegal and highly addictive substance. Understanding its structure, properties, and impact helps people see both the science and the risks behind it.