Table of Contents
- Chemical Differences & Why It Matters?
- Metabolism & Toxic Pathways
- Symptoms: Notice the Alarms
- How Much Is Dangerous?
- Diagnosis: How Doctors Spot It?
- Treatment: How to Combat the Poison?
- Why is Methanol Toxicity More Dangerous?
- Real‑World Risks & Outbreaks
- Prevention Tips
- Summary: Side-by-Side Comparison
- Which One Is Safer?
When comparing methanol and ethanol, we find that although both are types of alcohol, they affect our bodies in very different ways. Ethanol is the alcohol you find in drinks like beer, wine, and spirits. In contrast, methanol (also called wood alcohol) isn’t meant to be consumed. Even small amounts of methanol can be dangerous, while ethanol is relatively safe in moderate amounts. In this article, you’ll understand methanol toxicity, how it happens compared to ethanol, what signs to look for, how doctors treat it, and how to stay safe.
Chemical Differences & Why It Matters?
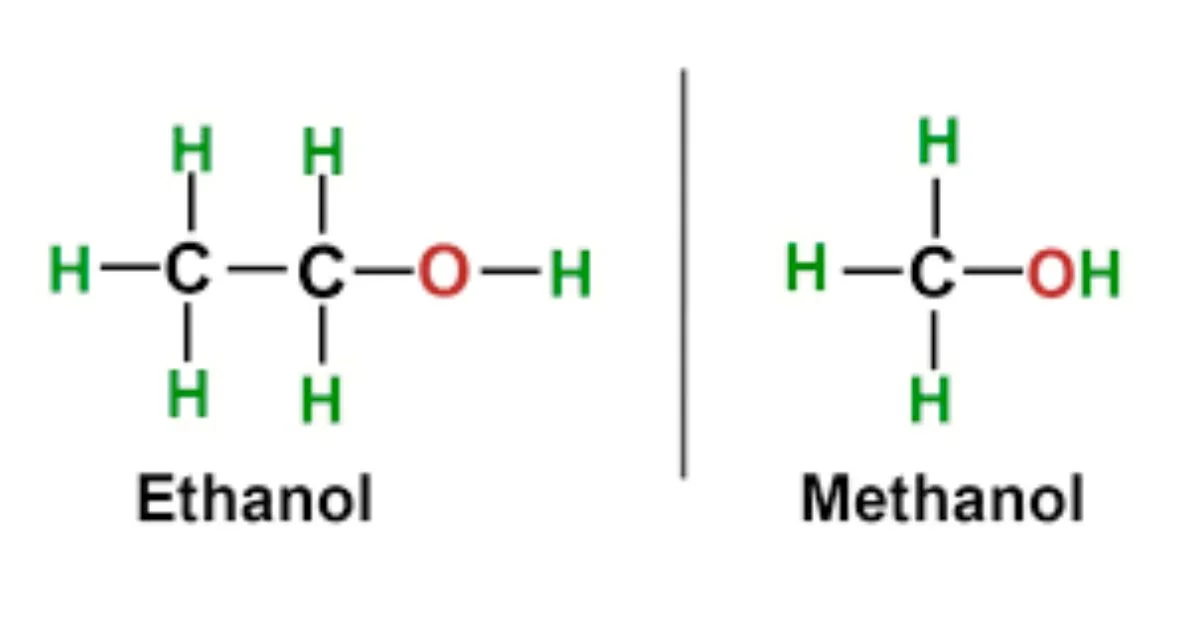
Ethanol (C₂H₅OH) contains two carbon atoms and is made naturally during fermentation. It’s legal to drink and relatively safe in small doses. But methanol (CH₃OH) has just one carbon atom and is made industrially, used in things like antifreeze, solvents, and fuel. While both are colorless, flammable liquids with an alcohol smell, methanol is notably more harmful because of how our bodies break it down.
Metabolism & Toxic Pathways
- Ethanol: IT is first converted by the enzyme alcohol dehydrogenase into acetaldehyde. Then, aldehyde dehydrogenase turns it into acetate. Acetate is fairly harmless; it’s a normal part of our metabolism.
- Methanol: However, it is converted into formaldehyde and then into formic acid. Formic acid builds up, causing blood acidity (metabolic acidosis), and can lead to nerve damage, especially in the eyes.
In simple terms, ethanol breaks down into something your body understands and uses, while methanol breaks down into toxic chemicals that harm critical organs.
Symptoms: Notice the Alarms
Ethanol (Typical Alcohol Intoxication)
- Mild impairment: Relaxed state, slowed judgment, slower reflexes.
- In large amounts: Vomiting, dizziness, unconsciousness, breathing issues, and in rare cases, death.
Methanol Toxicity
- Initial phase (1–12 hours): May feel a bit drunk, or even appear normal.
- Later phase (12–30 hours): Blurred or lost vision, headaches, nausea, stomach pain, deep breathing (hyperventilation), irregular heartbeat, low blood pressure, drowsiness, or coma.
- Serious complications: Metabolic acidosis, kidney failure, permanent blindness, seizures, and even death.
Vision problems are a hallmark of methanol poisoning, and the delay in symptoms often means victims don’t seek help until it’s too late.
How Much Is Dangerous?
| Alcohol Type | Blindness Dose | Fatal Dose |
|---|---|---|
| Methanol | ~10 mL | ~30–100 mL |
| Ethanol | Not typical | ~200 mL pure (~14 drinks) |
- Just 10 mL (2 tsp) of methanol can cause blindness, 30 mL (1 oz) could be fatal.
- By contrast, ethanol in typical drink sizes can hurt your liver but rarely causes blindness. The amount that kills someone is much higher.
Diagnosis: How Doctors Spot It?
Doctors identify methanol poisoning by looking for:
- High blood acidity (blood pH below 7.35).
- High anion gap metabolic acidosis.
- Elevated methanol levels in blood tests.
- Signs like fast breathing, fast heartbeat, confusion, and sudden vision loss.
They also ask about history, like exposure to bootleg alcohol or industrial products.
Treatment: How to Combat the Poison?
The goal is to prevent methanol from turning into toxic metabolites and remove them from the body quickly.
1. Antidotes
- Fomepizole: the drug of choice, it blocks alcohol dehydrogenase, stopping methanol from converting into harmful acids.
- Ethanol therapy: using alcohol to compete with methanol at the enzyme site. Less ideal but still effective when fomepizole isn’t available.
2. Bicarbonate
- Given to correct blood acidity (metabolic acidosis).
3. Dialysis
- Used in severe cases to remove methanol and formic acid from the blood, especially when vision is affected or blood acidosis is severe.
4. Supportive care
- Oxygen, IV fluids, breathing support, folinic acid to help break down formic acid, and close monitoring.
Timing is critical; treatment within the first 10–30 hours significantly improves outcomes.
Why is Methanol Toxicity More Dangerous?
Our bodies naturally deal well with ethanol but create toxins from methanol. Here are key reasons methanol is far deadlier:
- Toxic metabolites: Methanol becomes formaldehyde and formic acid. Formic acid blocks cell energy machines, causing pain and damage.
- Delayed symptoms: You may feel okay for hours after exposure, but serious damage is happening inside.
- Eye vulnerability: The optic nerve has lots of mitochondria, so formic acid damages the eyes first.
Real‑World Risks & Outbreaks
Methanol toxicity often happens in unsafe alcohol production:
- Illicit liquor: People are adding methanol to fake strong drinks because it’s cheap.
- Bootleg outbreaks: In 2016, 74–78 people died in Russia’s Irkutsk from a contaminated bath oil labeled as alcohol.
- Tourist cases: In Laos, six tourists died in late 2024 from methanol in local spirits.
Takeaway: Only drink from trusted sources. Be cautious with homemade or cheap alcohol, especially abroad.
Prevention Tips
- Buy only licensed, sealed beverages from reputable bars or shops.
- Avoid homemade liquor, common in traditional or rural settings.
- Report suspicious alcohol, especially if the labels look fake.
- Seek immediate help if you notice unusual symptoms after drinking, especially vision problems.
Summary: Side-by-Side Comparison
| Feature | Ethanol | Methanol |
|---|---|---|
| Source | Fermented drinks | Industrial solvent, lab use |
| Metabolism | Ethanol → acetaldehyde → acetate | Methanol → formaldehyde → formic acid |
| Toxic by‑products | Acetate (harmless at small doses) | Formic acid (damages cells & optic nerve) |
| Symptoms appearance | Immediate intoxication | May feel okay, followed by delayed crisis |
| Key danger | Intoxication, liver damage | Blindness, acidosis, coma, death |
| Treatment | Supportive care | Fomepizole or ethanol, bicarbonate, dialysis |
| Safe for drinking? | Yes, in moderation | No, dangerous even in tiny amounts |
Which One Is Safer?
In the end, both ethanol and methanol are types of alcohol, but their effects on the body are completely different. Ethanol is the kind found in alcoholic drinks and, when consumed in moderate amounts, is processed safely by the body. Methanol, on the other hand, is highly toxic, even small amounts can lead to blindness, brain damage, or death. The key difference lies in how they’re broken down: ethanol creates harmless by-products, while methanol turns into dangerous formic acid that harms your nerves and organs. So, when it comes to safety, ethanol is clearly the safer alcohol, but caution is always necessary. If there’s ever a chance methanol has been consumed, seek emergency medical help immediately, acting quickly can save a life.
Related Topic: Methyl Formate: Properties, Production, Uses, and Market Trends